Historically, scaling up from the laboratory to a pilot stage has been a crucial step before progressing to commercial production. This is due to several factors, including:
Retaining engineering expertise for process development is of utmost importance, as is establishing a theoretical comprehension of the process through a mathematical model. The demanding nature of the tasks faced by chemical engineers highlights the importance of dependable kinetic and sufficient mass transfer models, which are grounded in a physicochemical understanding of catalytic processes.
Understand your kinetics
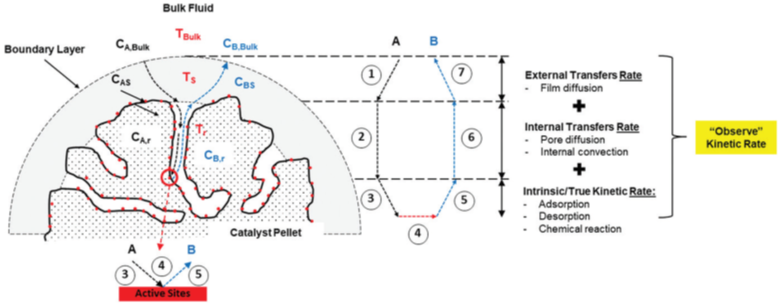
Catalytic kinetics under non-steady-state conditions are crucial, as catalysis inherently involves kinetic phenomena, and the rates and equilibria of reactions are essential for understanding reaction mechanisms. In industrial settings, rates of chemical transformations are often influenced by other processes, particularly mass and heat transfer. Quantitative analysis of kinetic and mass transfer phenomena becomes critical during the scale-up of laboratory processes to pilot scale.
Catalytic activity during pilot testing may differ from laboratory experiments. Catalytic kinetics aid in understanding activity, and models employing steady-state and quasi-equilibrium approximations based on elementary processes offer dependable extrapolations. It is essential to recognize that complex reaction kinetics are affected by the non-uniformity of catalyst surfaces, including size-dependent factors like cluster and reactant sizes. These effects must be addressed during the scale-up process.
Concentration, partial pressure, and activity variables in rate equations are crucial components of scale-up procedures and depend on whether the process involves a liquid-phase or gas-phase system. Designing data-gathering experiments for kinetic studies is vital, with attention to potential experimental errors and systematic deviations.
Consider effect of shape and size of your catalyst
Diffusion effects in heterogeneous catalysis and system kinetics must be considered. Modeling a two-phase catalytic reactor focuses on the catalyst particle, accounting for simultaneous reaction and diffusion within the particle’s pores. Inherent internal diffusion of reactants into porous catalyst particles must be addressed first, followed by external diffusion, which is reactor-dependent and involves mass transfer from the bulk to the catalyst surface.
Ensure your experiment and analysis
Through the analysis and experimental design of these complex variables, a research-based process can be scaled up to a larger pilot scale, enhancing the understanding of chemical, engineering, and economic phenomena. In related developments, microreactor technology has garnered significant interest in chemical engineering, increasingly contributing to the understanding of scaling up reactions from the laboratory to pilot stages.
Microreactor technology meets three fundamental requirements for chemical reactions: short residence time, heat introduction or removal, and sufficient mass transfer. Reactions can occur under more aggressive conditions with higher yields than conventional reactors. Moreover, new reaction pathways can be explored. Challenges with microreactors include introducing catalytic particles into reactors, necessitating considerable efforts in catalyst preparation methodology.
Apply reactor modelling for understanding effects of uncertainties
Reactor modeling for three-phase fixed-bed isothermal reactors encompasses the gas phase, liquid phase, and solid catalyst phase. Factors such as flow, dispersion effects, and interfacial fluxes must be considered for continuous fixed-bed reactors. Dynamic one-dimensional models have been established. In fine chemical manufacturing, semi-batch and batch reactors are used, equipped with auxiliary devices for mixing, heating, and cooling, as well as solvent distillation and evaporation. These reactors typically involve liquid-phase reactions with models focused on mass balances.