Decarbonization primarily involves transitioning from carbon-intensive energy sources, such as fossil fuels, to renewable and low-carbon alternatives. While the focus is often on technological advancements and policy measures, the underlying principles of thermodynamics play a crucial role in shaping the possibilities and limitations of this transition.
Thermodynamics, the science that deals with heat and energy, is fundamental to understanding how energy is produced, converted, and utilized across various systems and industries. The principles of thermodynamics govern everything from the efficiency of power plants and industrial processes to the performance of vehicles and the possibility of renewable energy technologies. These principles are not merely abstract scientific concepts; they are the real-world constraints and drivers that influence how effectively we can reduce carbon emissions.
Thermodynamics is central to the development and optimization of renewable energy technologies. Solar panels, wind turbines, and hydroelectric plants all operate based on thermodynamic principles. The efficiency with which these technologies convert natural forces into electricity determines their viability and capacity to replace fossil fuel-based energy.
1. Basic Principles of Thermodynamics Relevant to Decarbonization
it is needed to “understand the basic principles of thermodynamics that govern energy systems and their implications on efforts to reduce carbon emissions“. Thermodynamics, at its core, is concerned with the laws governing heat, energy, and their transformations. These principles are not just academic; they are the underpinnings of how we can harness, convert, and use energy in more sustainable ways.
The First Law of Thermodynamics – Conservation of Energy
This law, also known as the law of energy conservation, states that energy can neither be created nor destroyed; it can only be transformed from one form to another. In the context of decarbonization, this principle reminds us that all energy used must come from somewhere and eventually goes somewhere, often in the form of heat. It highlights the importance of maximizing energy efficiency and minimizing wasteful energy transformations in processes such as electricity generation, industrial manufacturing, and transportation
The Second Law of Thermodynamics – Entropy and Efficiency
The second law introduces the concept of entropy, a measure of disorder or randomness in a system. It states that in any energy conversion process, some energy is lost as waste heat, increasing the total entropy of the system. This law is crucial in understanding the inherent “limitations in energy conversion processes“. For example, it limits the maximum efficiency of heat engines and power plants. In decarbonization, this translates to a focus on technologies and processes that minimize energy loss and maximize the productive use of energy
Exergy analysis is a thermodynamic analysis technique based on the Second Law of Thermodynamics which provides an alternative and illuminating means of assessing and comparing processes and systems rationally and meaningfully. In particular, exergy analysis yields efficiencies, which provide a true measure of how nearly actual performance approaches “the ideal and identifies the causes and locations of thermodynamic losses more clearly than energy analysis”. Consequently, exergy analysis can assist in improving and optimizing designs. In decarbonization, exergy analysis can be used to assess the efficiency of energy systems and identify where improvements can be made to reduce energy waste and carbon emissions
2. The Thermodynamic Challenges in Energy Production
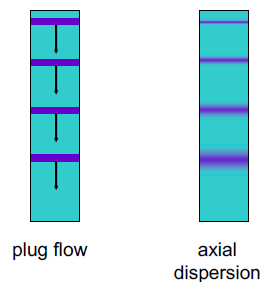
The limitations are imposed by the laws of thermodynamics, especially the Second Law. The maximum efficiency of heat engines (like those in power plants) is limited by the Carnot efficiency, which is dependent on the temperature difference between the heat source and the sink. Achieving high temperatures for the heat source and low temperatures for the sink is challenging, and even under ideal conditions, 100% efficiency is impossible. A significant portion of the energy (such as coal, natural gas, or nuclear material) is lost as waste heat.
Various thermodynamic cycles, such as the Carnot cycle, Rankine cycle, and Brayton cycle, are used to model the operation of heat engines, turbines, and other energy-conversion devices. Understanding these cycles helps in analyzing and improving the efficiency of power generation systems
One of the most common examples of efficiency limits in heat engines, especially in power plants, is the steam turbine system used in thermal power plants. The efficiency of such a system can be estimated using the concept of Carnot efficiency, which sets the theoretical maximum efficiency based on the temperatures of the heat source and sink as example:
Example: Steam Turbine in a Thermal Power Plant
A steam turbine in a thermal power plant takes in steam at a high temperature from a boiler and exhausts it at a lower temperature to a condenser,Let’s assume
Carnot Efficiency Calculation:
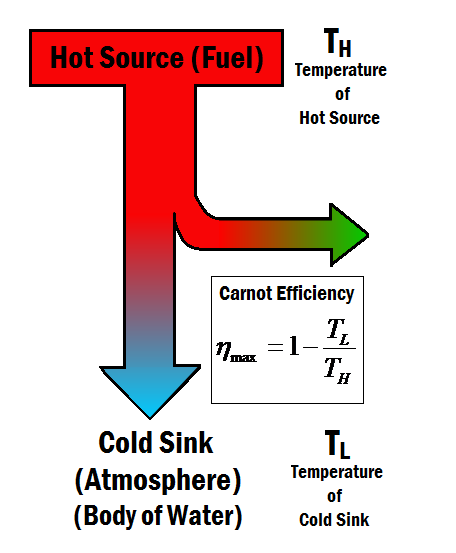
The Carnot efficiency for the steam turbine in this thermal power plant scenario is approximately 51.75%. This means that, theoretically, a maximum of about 51.75% of the heat energy can be converted into work.
When converting heat into work, some energy must always be “wasted” or unrecoverable, typically as heat dumped into a cooler reservoir. This is because converting heat to work requires a temperature gradient (a hot source and a cold sink), and the process invariably increases the entropy of the cold sink.
In real-world implications, the practical limitations, “the actual efficiency of steam turbines and other heat engines in power plants is lower than the Carnot efficiency”. This is due to additional real-world factors such as friction, heat losses, and other inefficiencies in the system. Engineers use these theoretical efficiency limits as benchmarks when designing and improving power plant systems (“achieving Carnot efficiency is impossible”).
3. Thermodynamic Constraints in CO2 conversion
The fundamental limitations imposed by the laws of thermodynamics on the conversion of carbon dioxide (CO2) into other useful products or for its sequestration.
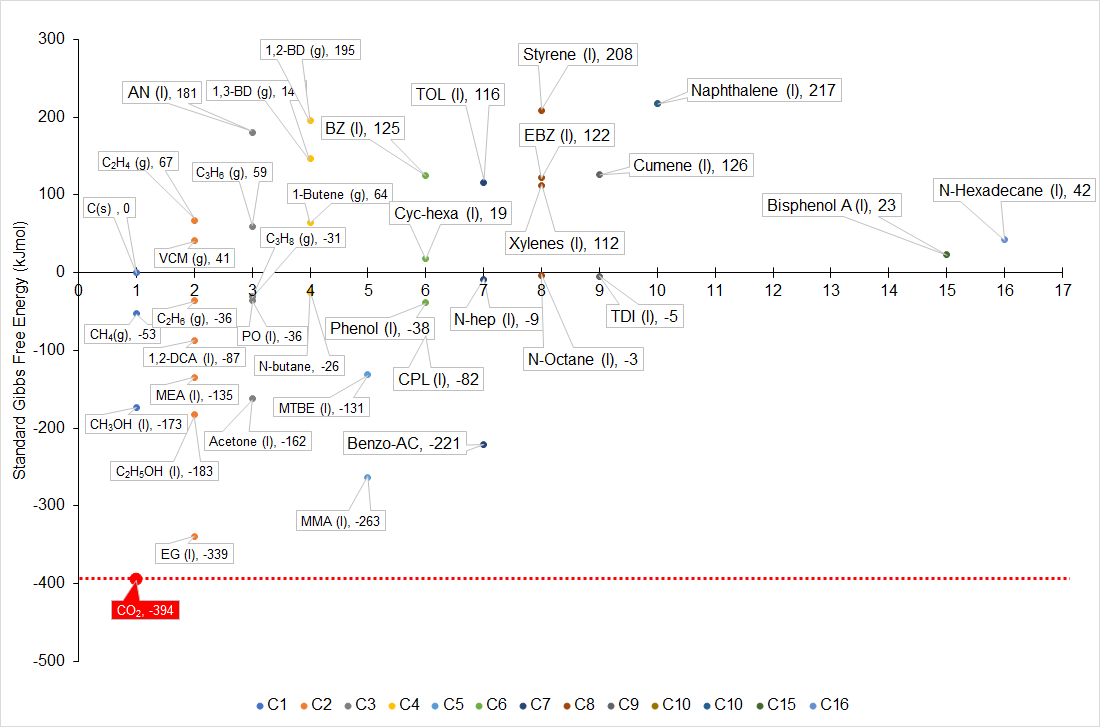
Gibbs Free Energy is a critical concept in understanding the thermodynamics of CO2 conversion processes. It’s a measure that combines the concepts of enthalpy (heat energy) and entropy (disorder) to determine the spontaneity and feasibility of chemical reactions. In the context of CO2 conversion, Gibbs Free Energy helps in assessing whether a given reaction can occur naturally and what external inputs (like energy or catalysts) might be necessary. According to the Second Law, processes tend to increase the entropy (disorder) of the system. CO2 conversion often involves reducing the entropy (creating more ordered structures), which is naturally unfavorable and requires external energy input. Many chemical reactions that convert CO2 into valuable chemicals are endothermic, meaning they require an input of energy ((typically involves a positive ΔG, meaning these reactions are non-spontaneous))
Carbon dioxide (CO2) is a thermodynamically stable molecule, especially in its gaseous form at standard temperatures and pressures. Compared to other industrial feedstocks, CO2 has a lower energy level, meaning it requires significant energy input to engage in chemical reactions. The energy needed to transform CO2 into various chemicals depends on the change in the oxidation state of the carbon atom. (Figure 2). For instance, when CO2 is used to create compounds like carboxylates, carbonates, and carbamates, where its oxidation state remains unchanged, the required energy input is relatively low. In contrast, producing chemicals like methanol or methane, which involves reducing CO2 by adding hydrogen, demands a much higher energy input for both the process heat and hydrogen generation.
Catalysts work by providing an alternative reaction pathway with a lower activation energy. This means that it takes less energy to initiate the reaction, making it proceed faster. While catalysts can speed up a reaction, they do not alter the initial or final energy states of the reactants and products. Thus, the overall Gibbs Free Energy change (ΔG) for the reaction remains the same. Catalysts can also influence the selectivity of a reaction, favoring the formation of certain products over others. This is particularly important in CO2 conversion processes where multiple products are possible. for “Thermodynamic vs. Kinetic Control”:
In summary, The role of catalysts in chemical reactions, including CO2 conversion processes, is critical, but it’s important to clarify that catalysts do not change the Gibbs Free Energy (ΔG) of the reaction. Instead, they affect the reaction kinetics – that is, they change the rate at which the reaction proceeds to its equilibrium state.
Thermodynamic Challenges in Capturing, Transporting, and Storing CO2
The commercial advancement of Carbon Capture and Storage (CCS) technologies has been significantly hindered by high costs. The development of Carbon Capture, Utilization, and Storage (CCUS) projects hinges on several critical factors, including the costs involved, available incentives, and public perception. In large-scale CCS projects, the captured CO2 is typically transported either by pipelines or ships. The storage of this captured CO2 is generally in depleted oil fields or saline aquifers. However, for the development of CCS projects on a larger scale, there are challenges that need addressing, particularly in managing and transporting CO2 cost-effectively, especially when it contains impurities. Additionally, many thermodynamic aspects of CO2 management are still under active discussion and research, indicating that this field is evolving and many questions remain to be answered.
Capturing, Transporting, and Storing CO2
Energy Requirements and Efficiency Trade-Offs
Term “energy penalty” refers to the additional energy required to operate a carbon capture process. The energy penalty measures the extra energy expenditure needed for carbon capture relative to the total energy output of the plant. It can be viewed in two ways: as a relative increase in the energy input, or as a relative decrease in the power output of a plant equipped with carbon capture technology, compared to the same plant operating without such technology.
For power plants, implementing CO2 capture technologies could lead to a significant “energy penalty.” This means that a portion of the energy generated by the plant must be diverted to operate the capture process, reducing the overall efficiency of the plant.
Designing new plants with integrated CO2 capture is more energy-efficient than retrofitting existing plants. However, retrofitting is often necessary due to the large number of existing emission sources.
The efficiency trade-offs in the transport and storage of CO2 involve balancing the energy requirements, costs, and environmental impacts of different transportation methods and storage options. Optimizing these aspects requires a comprehensive understanding of the various factors influencing each stage of the CCS process.
The choice between pipeline and shipping for CO2 transport involves trade-offs in terms of energy use, cost, and feasibility, depending on the distance and terrain. Pipelines is the most common method for transporting large volumes of CO2 is via pipelines. Pipelines are generally efficient for continuous, large-scale transport over land. In cases where pipelines are not feasible, such as for offshore storage sites or across difficult terrain, CO2 might be transported by ships. Shipping is more flexible but can be less energy-efficient and more costly for large volumes.
Compressed or Liquefied CO2 is typically transported in a compressed or liquefied state, which requires energy for compression and, in some cases, refrigeration to maintain the necessary temperature and pressure conditions.
The injected carbon dioxide can be stored in the formation of the reservoir. Injecting CO2 into geological formations requires energy, primarily to maintain the pressure necessary for injection. The amount of energy depends on the depth and type of the geological formation.
Options of Storage Sites include depleted oil and gas fields, deep saline aquifers, and unmineable coal seams. Each type has different characteristics affecting the ease and efficiency of injection.
Storage Efficiency Factors might be roughly site accessibility and permeability and capacity. Sites that are more accessible and closer to the source of CO2 emissions generally require less energy for transport and injection. The permeability and storage capacity of the geological formation impact the energy required for injection. Higher permeability and larger capacity can reduce the energy needed.
In term of leakage risks, there is a trade-off between the energy used to ensure secure storage (like in deeper, more stable formations) and the risk of leakage, which would negate the environmental benefits of storage.
The additional cost for capturing, transporting, and storing CO2 must be weighed against the environmental benefits of reduced atmospheric CO2 emissions. Each stage of the CCS chain (capture, transport, and storage) has its own efficiency trade-offs. Optimizing the entire CCS chain involves balancing these different factors to achieve the lowest overall cost and highest environmental benefit. Proximity to emission sources and integration with emission sources of industrial processes can significantly impact the efficiency and feasibility of the CCS chain.
In summary, the efficiency trade-offs in the transport and storage of CO2 involve balancing the energy requirements, costs, and environmental impacts of different transportation methods and storage options. These trade-offs are crucial in determining the overall viability and sustainability of CCS projects. Optimizing these aspects requires a comprehensive understanding of the various factors influencing each stage of the CCS process.
Thermodynamic Principles in Waste Heat Recovery Systems
Thermodynamic principles in waste heat recovery systems are fundamental in understanding how these systems capture and reuse heat that would otherwise be lost to the environment. These principles are based on the laws of thermodynamics, which govern energy transformations and efficiency.
The Table 1. provides an overview of different waste heat recovery technologies, highlighting their typical use-cases and the balance between benefits and limitations.
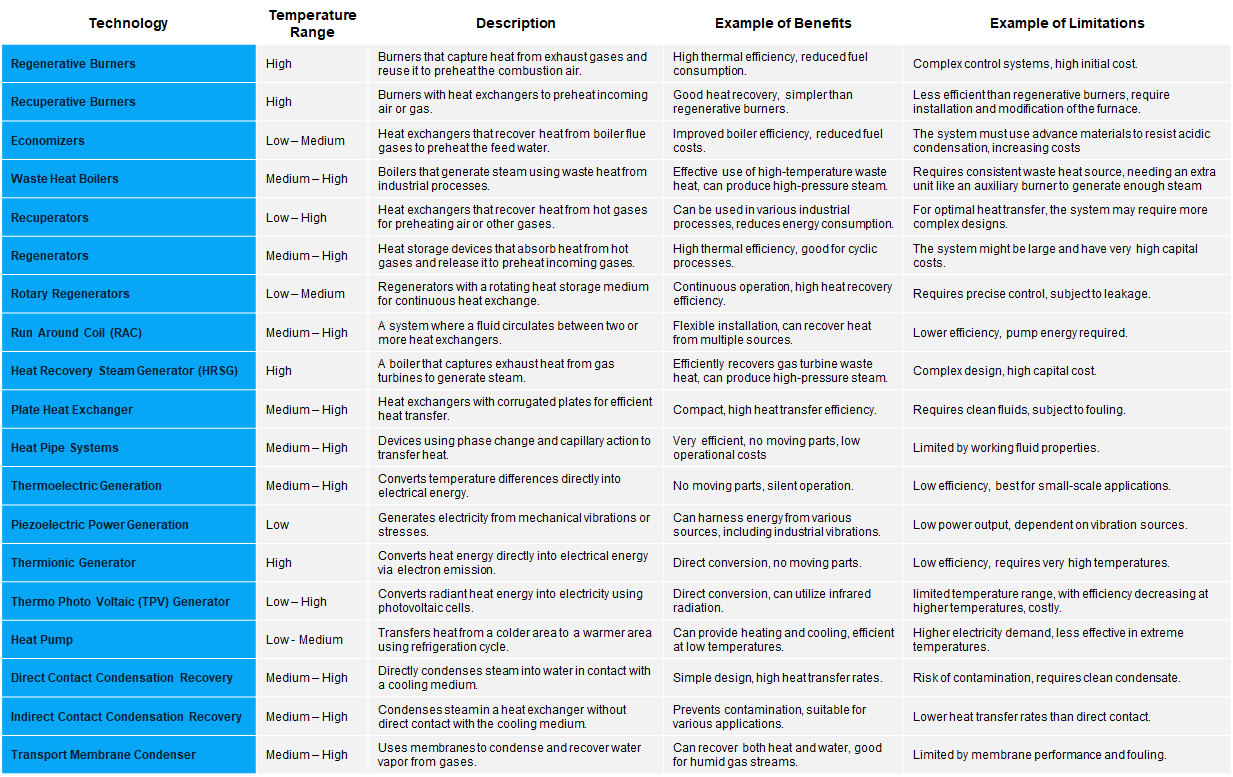
Waste heat recovery involves capturing and transferring waste heat from a process back into the system as an additional energy source. The energy source can be utilized to produce extra heat or to generate electrical and mechanical power. While waste heat can be emitted at any temperature, typically, higher temperatures mean better quality waste heat, making the recovery process easier to optimize. Therefore, it’s vital to identify the maximum recoverable heat of the highest quality from a process to ensure maximum efficiency in a waste heat recovery system.
Key Takeaways
Importance of Thermodynamic Principles in Decarbonization:
Disclaimer:
The views and opinions expressed in this Linkedin article are solely my own and do not represent the views or opinions of my current employer. This article is a personal reflection and does not involve any proprietary or confidential information from my current company. Any similarities in ideas or concepts presented in this article to my current company’s work are purely coincidental.